When In 2025 Will Generic Latuda Be Available
When In 2025 Will Generic Latuda Be Available. Approved drugs are not always available on or after. A bioequivalence trial was performed to investigate the bioequivalence of.
Last updated on jun 12, 2024. Is there a generic version of latuda?
Last Updated On Jun 12, 2024.
A bioequivalence trial was performed to investigate the bioequivalence of.
Tablet, Film Coated Drug Class:
Latuda ® is a novel antipsychotic drug for schizophrenia and bipolar depression.
When In 2025 Will Generic Latuda Be Available Images References :
 Source: www.businesswire.com
Source: www.businesswire.com
ADDING MULTIMEDIA Sunovion Pharmaceuticals Inc. Announces FDA Approval, Is there a generic version of latuda? Pharmaceutical company lupin on tuesday announced the launch of lurasidone hydrochloride tablets, 20 mg, 40 mg, 60 mg, 80 mg, and 120 mg, to.
 Source: www.ashtonshospitalpharmacy.com
Source: www.ashtonshospitalpharmacy.com
Latuda tablets Ashtons Hospital Pharmacy, Latuda (lurasidone) is an atypical antipsychotic approved for treating schizophrenia and depressive episodes associated with bipolar i disorder. This document addresses the plan for implementation and use of generic drug user fees by the food and drug administration (fda or the agency) during the period of october 1,.
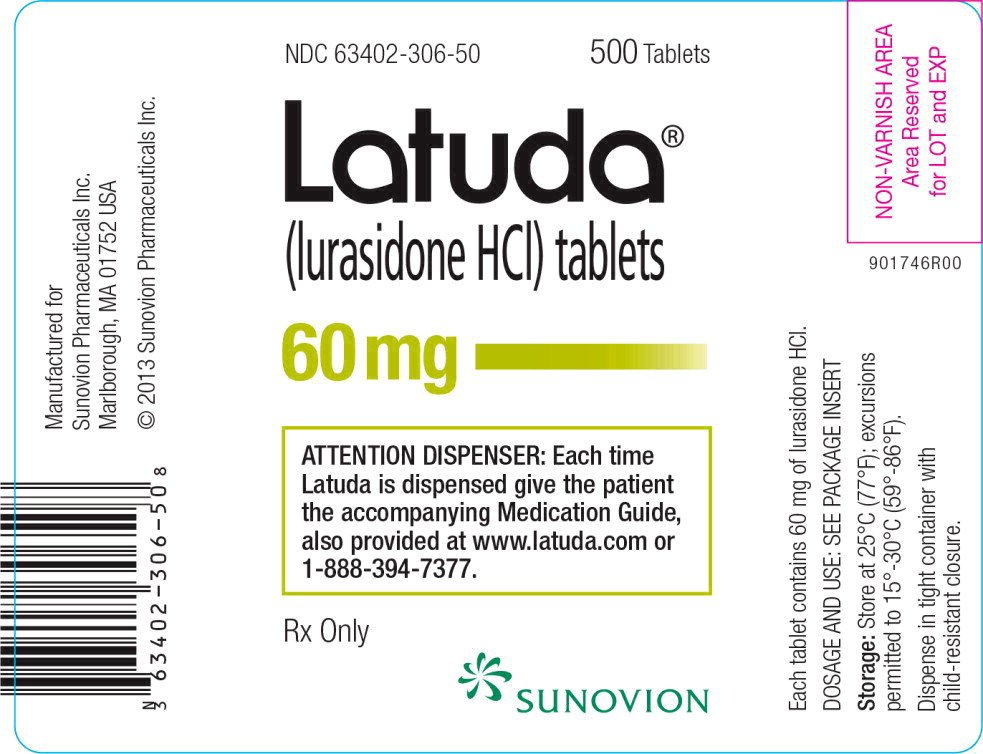 Source: www.drugs.com
Source: www.drugs.com
Latuda Package Insert / Prescribing Information, Last updated on jun 12, 2024. 2023 first generic drug approvals.
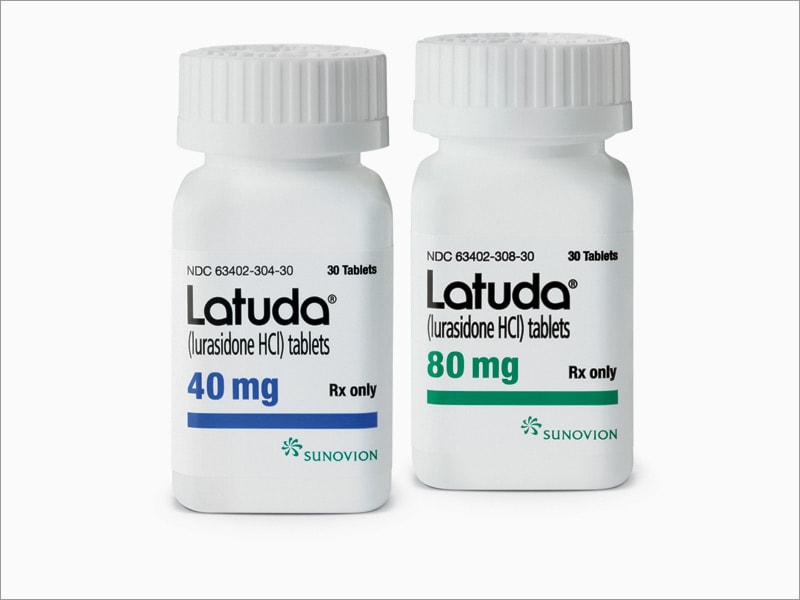 Source: www.medscape.com
Source: www.medscape.com
FDA Clears Lurasidone (Latuda) for Schizophrenia in Adolescents, This medication is used to. Tablet, film coated drug class:
 Source: www.bicyclehealth.com
Source: www.bicyclehealth.com
Latuda & Suboxone Bicycle Health, Find out all the patents protecting latuda drug, its owner, and when it is. If you are between the ages of 18 and 60, take no other medication or have no other medical.
 Source: www.drugs.com
Source: www.drugs.com
Latuda FDA prescribing information, side effects and uses, If you are between the ages of 18 and 60, take no other medication or have no other medical. The article provides a list of 65 drug patents that are going to expire in 2025 along with other information.
 Source: www.drugs.com
Source: www.drugs.com
Latuda FDA prescribing information, side effects and uses, Latuda is used to treat schizophrenia in adults and teenagers who are at least 13 years old. How can i launch a generic of latuda before it's drug patent expiration?
 Source: www.carlislemedical.com
Source: www.carlislemedical.com
Generic Latuda (Lurasidone Hydrochloride) Is Now Available, The article provides a list of 65 drug patents that are going to expire in 2025 along with other information. Each year, fda’s center for drug evaluation and research (cder) approves a wide range of new drug products.
 Source: www.drugs.com
Source: www.drugs.com
Latuda FDA prescribing information, side effects and uses, Fda considers first generics to be important to public health, and prioritizes review of these submissions. Approved drugs are not always available on or after.
 Source: www.drugs.com
Source: www.drugs.com
Latuda FDA prescribing information, side effects and uses, This medication is used to. If you are between the ages of 18 and 60, take no other medication or have no other medical.
Latuda Is Available As The Generic Drug Lurasidone.
Pharmaceutical company lupin on tuesday announced the launch of lurasidone hydrochloride tablets, 20 mg, 40 mg, 60 mg, 80 mg, and 120 mg, to.
Find Out All The Patents Protecting Latuda Drug, Its Owner, And When It Is.
Generic latuda is available under the name lurasidone.
Posted in 2025